electron geometry of ch4|ch4 hybridization : Cebu In this video we look at the electron geometry for Methane (CH4). Because the methane molecule has four electron domains (four hydrogen atoms and no lone pairs) the electron geometry will be. While playing online keno, having a clear understanding of the game’s associated odds and payouts is important. The odds of winning in keno depend on how many numbers you pick and catch, with the chances of winning varying based on your selections. For example, if you pick one number, your odds are 1 in 4, but if you pick four numbers, your .
PH0 · po4 3 electronic geometry
PH1 · electron geometry vs molecular geometry
PH2 · electron geometry chart
PH3 · electron and molecular geometry table
PH4 · ch4 lewis structure
PH5 · ch4 hybridization
PH6 · ch4 bond angle
PH7 · Iba pa
Hotel Beauchamps, Paris: 699 Hotel Reviews, 324 traveller photos, and great deals for Hotel Beauchamps, ranked #814 of 1,900 hotels in Paris and rated 4 of 5 at Tripadvisor.
electron geometry of ch4*******In this video we look at the electron geometry for Methane (CH4). Because the methane molecule has four electron domains (four hydrogen atoms and no lone pairs) the electron geometry will be. An explanation of the molecular geometry (and Electron Geometry) for the CH4 (Methane) including a description of the CH4 bond angles. The electron geometry . Learn how to draw the lewis structure of methane (CH4) and understand its geometrical and hybridization properties. The web page explains the valence electrons, octet rule, VSEPR theory, and .
Valence Shell Electron Pair Repulsion. Methane has 4 regions of electron density around the central carbon atom (4 bonds, no lone pairs). The resulting shape is a regular tetrahedron with H-C-H angles of 109.5°.The bonding in the hydrogen molecule is fairly straightforward, but the situation is more complicated in organic molecules with tetravalent carbon atoms. Take methane, CH 4, . A quick explanation of the molecular geometry for CH4 including bond angle, hybridization, and polarity of CH4.Helpful Resources:• How to Draw Lewis Structur. Two "election clouds" or regions of electron density around a central atom in a molecule form a linear geometry; three electron clouds form a trigonal planar .
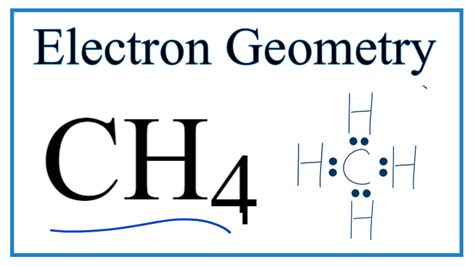
Structure of methane. CH 4 contains 4 bonded and no nonbonded electron domains, giving tetrahedral e - domain and molecular geometries. (AX 3 E 1 ). The H-C-H bond angles .electron geometry of ch4 What is the proposed molecular geometry for CH4? Let’s begin by drawing a Lewis structure for methane. To determine the molecular geometry or shape of this .
Thus, the electron-pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109.5°. In fact, the bond angle is 104.5°. Figure \(\PageIndex{9}\): (a) H 2 O has four regions of .
As stated above, molecular geometry and electron-group geometry are the same when there are no lone pairs. The VSEPR notation for these molecules are AX n. "A" represents the central atom and n represents the number of bonds with the central atom. When lone pairs are present, the letter E x is added. The x represents the number . Figure 5.9.5 5.9. 5: (a) The electron-pair geometry for the ammonia molecule is tetrahedral with one lone pair and three single bonds. (b) The trigonal pyramidal molecular structure is determined .
A quick explanation of the molecular geometry of CH4 including a description of the CH4 bond angles.Looking at the CH4 Lewis structure we can see that there .
Electron-pair Geometry versus Molecular Structure. It is important to note that electron-pair geometry around a central atom is not the same thing as its molecular structure. The electron-pair geometries shown in Figure 7.16 describe all regions where electrons are located, bonds as well as lone pairs. Molecular structure describes the location of the .Trigonal Bipyramidal Electron Geometry. A central atom with five pairs of bonding electron pairs is known as trigonal bipyramidal. It has the shape of three pairs in a plane at 120° angles (the trigonal planar geometry) and the remaining two pairs at 90° angles to the plane. The shape is similar to two pyramids joined by a triangular base. 1. The central atom, sulfur, contributes six valence electrons, and each fluorine atom has seven valence electrons, so the Lewis electron structure is. With an expanded valence, that this species is an exception to the octet rule. 2. There are six electron groups around the central atom, each a bonding pair.
CH4 lewis structure shape. According to the VSEPR (Valence Shell Electrons Pair Repulsion) theory if the electrons count of any molecule is 8 then the molecule adopts tetrahedral geometry. The electrons contribution for C is 4 and four H atoms contribute 1 electron each, so the total electron count will be 8. So, the CH4 lewis structure is .
We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom, ignoring all other valence electrons present.According to this model, valence electrons in the Lewis structure form groups, which may consist of a single bond, a double bond, a triple bond, .
ch4 hybridization CH4 Molecular Geometry. Methane has a tetrahedral molecular geometry with bond angles of 109.5°. There are four covalent bonds between hydrogen atoms and central carbon atoms in the methane molecule. According to the Valence shell electron pair repulsion (VSEPR) theory, the same kind of electron pair repels each other. As there .The molecular geometry of CH4 is tetrahedral. This means that the four hydrogen atoms are arranged around the central carbon atom in a way that forms a pyramid-like shape. . The valence electron count determines the number of electrons an atom can share with other atoms to form covalent bonds. In the case of CH4, the carbon atom has four .The electron geometry of CH4 can be determined using the VSEPR (Valence Shell Electron Pair Repulsion) theory, which considers all electron pairs around the central atom, including both bonding and .

CH 4 contains 4 bonded and no nonbonded electron domains, giving tetrahedral e-domain and molecular geometries. (AX 3 E 1).The H-C-H bond angles (109.5 degrees) are those predicted for a perfect tetrahedral geometry. Use your mouse (computer) or fingers (touch screen) to manipulate the Jmol model.
electron geometry of ch4 ch4 hybridization Electron configuration of \(\ce{C}\) atom is: 1s 2, 2s 2, 2p x 1, 2p y 1, 2p z 0, where 1s is the core-shell and 2s and 2p are in the valence shell. . This difference between the actual and the expected geometry of \(\ce{CH4}\) molecule is explained by the orbital hybridization concept.The electron group geometry for a molecule with four electron pairs is tetrahedral, as was seen with \(\ce{CH_4}\). In the ammonia molecule, one of the electron pairs is a lone pair rather than a bonding pair. Although the lone pair is not visible, it will affects the location and bond angles among other atoms in the molecule.
Ammonia, NH3, is a pyramid-shaped molecule, with the hydrogens in an equilateral triangle, the nitrogen above the plane of this triangle, and a H-N-H angle equal to 107°. The geometry of CH4 is that of a tetrahedron, with all H-C-H angles equal to 109.5°. (See also Figure.) Ethane, C2H6, has a geometry related to that of methane.We recommend using the latest version of Chrome, Firefox, Safari, or Edge. Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules!
Slot, sport betting, fishing. Easy register & login. 24/24 withdraw by Gcash, Paymaya,.. Skip to content. WinPH Apps Download . Tara na, mag-register na sa WinPH at mag-enjoy sa mas maraming halakhak at kita! WinPH Login – Sumali sa Pilipinas Swerte, Kumuha ng Daily Sign-In Bonus Paano Makukuha ang Daily Sign-In Bonus ng WinPH: .
electron geometry of ch4|ch4 hybridization